Mass Formula Chemistry
Mass number of moles relative formula mass 2 44 88 g Finding the relative formula mass Question. What is the formula mass amu of this compound.
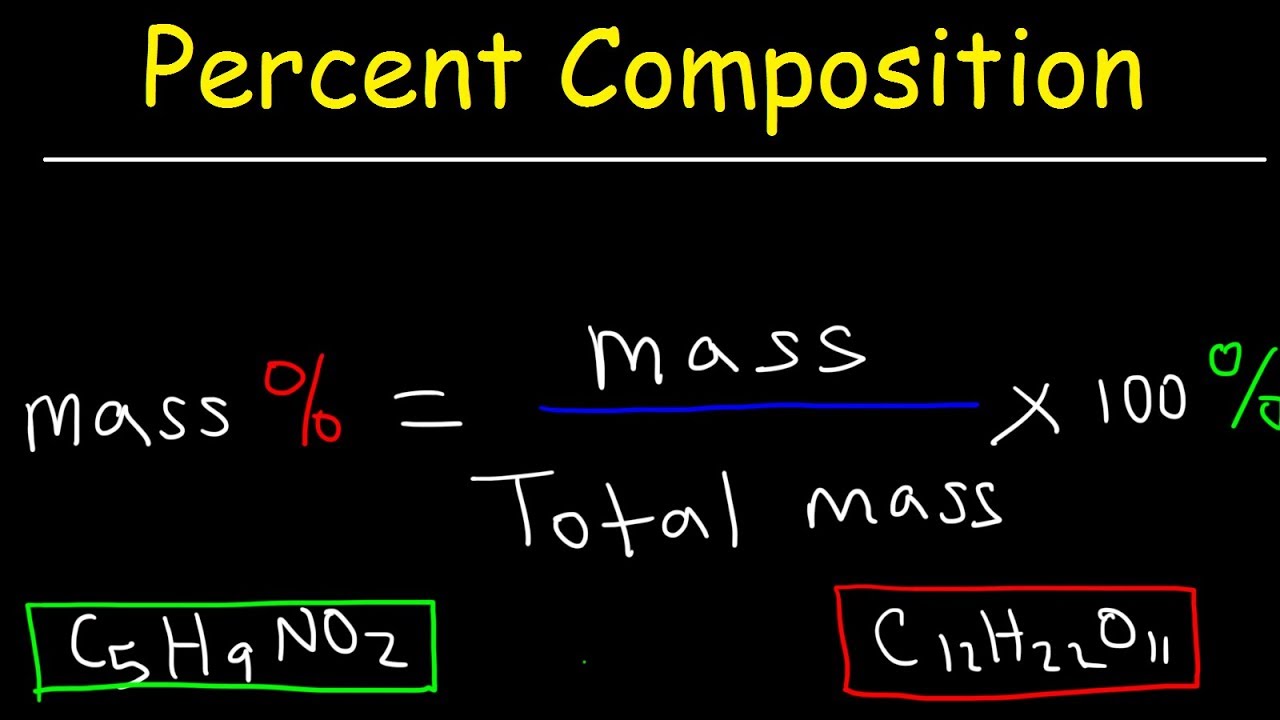
Chemistry Lesson Percent Composition Youtube Chemistry Lessons Teaching Chemistry Ap Chemistry
Find the element Mass number whose atomic number is 15 and the number of neutrons present is 15.

. All The Learning Tools You Need In One Place. Make use of the chemical formula to determine the number of atoms of each element in the compound. Mass by Mass Mass of soluteMass of Solution 100.
Mass percent composition describes the relative quantities of elements in a chemical compound. The formula for this compound indicates it contains Al 3 and SO 4 2 ions combined in a 23 ratio. A syrup of 2125g mass has 34g mass of sugar.
The molar mass M is a physical property and it is defined as the mass of one mole of the chemical substance or it is a ratio of the mass of a chemical compound to its amount of. Ad Learn Chemistry Concepts With Our Practice Tests Study Guides Video Lessons. 10 mol of carbon dioxide has a mass of 440 g.
Calculating the Mass Density of a Substance. The average mass of a chloroform molecule CHCl3 CHCl 3 is 11937 amu which is the sum of the average atomic masses of each of its constituent atoms. For purposes of computing.
Molar Mass Formula is the sum of the atomic mass of all the. The formula mass of aluminium sulphate is 34217 u and its relative formula mass is 34217. Identify the mass of the solute.
Following the approach outlined above the formula mass for this. Molar Mass Formula is defined as the mass of a given element divided by the number of moles in that element. Multiply the atomic weight of each element with its number of atoms.
Calculate the formula mass of Carbon i Oxide. This can be calculated using the formula for molar. Start Earning Better Grades Today.
Identify the volume of solution. Determine the sugar concentration in the syrup given. Mass percent composition is also known percent by weight.
For purposes of computing a formula mass it is helpful to rewrite the formula in the. The atomic mass of carbon is 12 while the atomic mass of. The ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constant is defined as relative atomic mass A r or atomic weight.
Carbon i Oxide is a compound made of two element. Divide the mass of the solute by the volume of solution to find the. For purposes of computing a formula mass it is helpful to rewrite the formula in the simpler format Al 2 S 3 O 12.
The formula for this compound indicates it contains Al 3 and SO 4 2 ions combined in a 23 ratio. To Determined Formula Mass of Copper Sulphate Pentahydrate. Read the given information carefully and note the value of the mass of the substance and the value of the volume of the substance.

Calculating Average Atomic Mass Chemistry Lessons Chemistry Classroom Teaching Chemistry

Difference Between Formula Mass And Molecular Mass Comparison Summary Chemistry Lessons Teaching Chemistry Chemistry Education

Step 25 Mass Molar Mass And Mole Relationship 100 Steps To Sat Ii Chemistry From Unisprint Sat Satchem Chemistry Lessons Chemistry Ap Chemistry

Formula Triangle Molarity Teaching Chemistry Chemistry Classroom Chemistry Lessons
0 Response to "Mass Formula Chemistry"
Post a Comment